Image credit: Unsplash
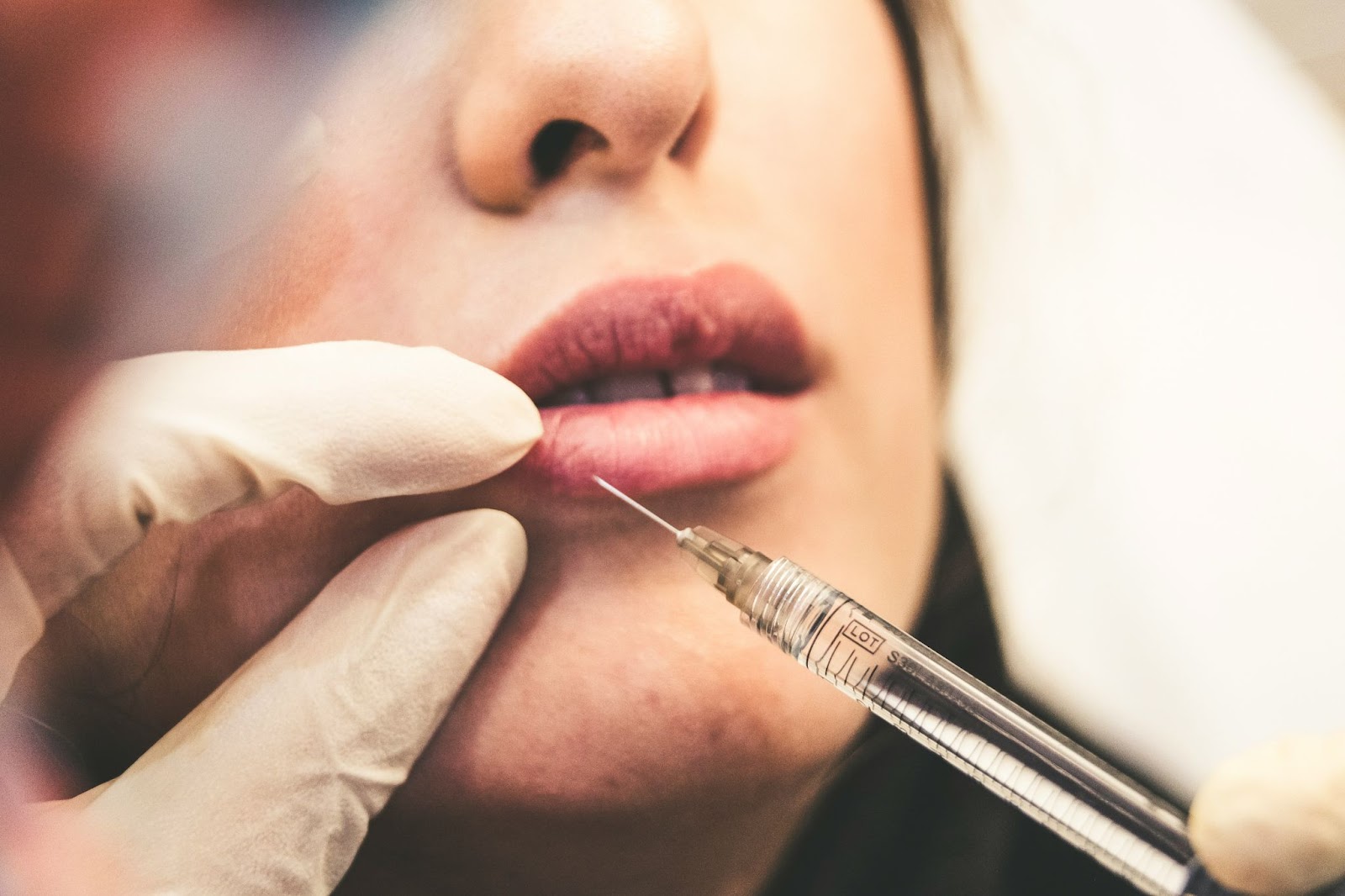
On Wednesday, a warning was issued by officials with the California Department of Public Health (CDPH), advising healthcare providers and consumers seeking Botox injections that reports of counterfeit Botox have been verified in several states.
Warnings from Health Officials About Counterfeit Botox
The CDPH released a statement detailing how injections of the counterfeit product were determined to have been administered in unlicensed and non-medical settings. This statement continued to explain that fake Botox injections have already caused hospitalizations for several severe reactions. The U.S. Centers for Disease Control and Prevention (CDC) spoke about counterfeit Botox last month, saying that injections had sickened at least 22 people in 11 states. California was among the states specified in that report.
In another statement, California Public Health Officer, Dr. Tomas Aragon, stated, “Counterfeit or incorrectly administered Botox, even in small amounts, can result in serious health problems and even death.”
Officials with the CDPH stated that the agency is working intimately alongside both the CDC and the U.S. Food and Drug Administration (FDA), as well as several other state health departments. This is being done as part of the FDA’s continuing investigation into the matter.
In the meantime, Dr. Aragon has publicly urged consumers who are seeking Botox injections to have the procedure done in a certified healthcare setting, administered by licensed and trained professionals. Consumers are being warned to never purchase Botox through any unlicensed individuals or online platforms.
According to health officials, California permits Botox treatments to only be performed by a licensed physician, a physician assistant under the supervision of a verified doctor, or a registered nurse. If any individual seeking injections has doubts or concerns about an injection, they are advised not to get the Botox shots performed and to report their concerns.
The CDPH released several details to help consumers verify Botox injections, stating that authentic Botox is manufactured solely by AbbVie Inc. According to the CDPH, the FDA-approved product lists the active ingredient as “OnabotulinumtoxinA,” which is the name that will be listed on the outer cartoon vile of approved Botox. No consumers who have used the FDA-approved product have reported any adverse health effects.
Consumers are urged to be on the lookout for the counterfeit product which is labeled, “Botulinum Toxin Type A,” and comes in 150-unit doses. The counterfeit Botox vials contain the lot number C3709C3.
Reports show that the symptoms of illness caused by counterfeit Botox are similar in nature to those exhibited by people who have suffered Botulism poisoning. These symptoms include difficulty swallowing, drooping eyelids, slurred speech, dry mouth, fatigue, difficulty breathing, and general weakness.
Symptoms and Safety Measures for Consumers
Anyone who has recently taken Botox and is experiencing any of the above-listed symptoms, or anyone experiencing any general symptoms of Botulism poisoning, is urged to go to the emergency room immediately or contact their healthcare provider as soon as possible.
Consumers who suspect counterfeit Botox products can file a report through the FDA’s website or by calling 1-800-551-3989. Consumers can also send reports to the consumer complaints section of the CDPH website.